Abstract
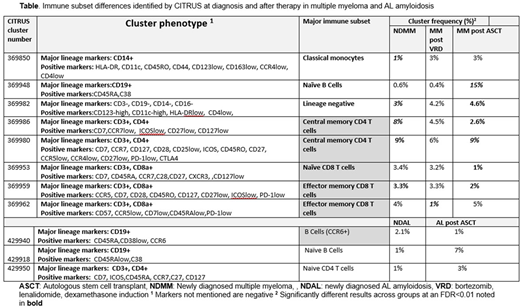
INTRODUCTION: Traditional cytometry methods have been unable to capture the immense heterogeneity of the tumor immune microenvironment in various malignancies including multiple myeloma (MM). Cytometry by time of flight (CyTOF) can, to some extent, overcome this limitation. However, the computational challenges that come with analyzing these complex datasets in a reproducible manner remain. In this study, using a large cohort of patients, we compare the bone marrow immunomes from patients with MGUS, multiple myeloma (MM), smoldering MM (SMM) and light chain amyloidosis (AL) at diagnosis, after induction therapy with lenalidomide and dexamethasone and after autologous transplant (ASCT). METHODS: We studied a total of 118 cryopreserved samples as follows: 14 healthy donors, 43 AL (27 newly diagnosed-ND, of which 13 with <10% bone marrow plasma cells, 16 matched samples post ASCT), 12 with ND MGUS, 11 with SMM (of which 6 were ND), 14 with ND MM, 13 paired MM samples post induction therapy and 11 paired MM samples post ASCT. Our CyTOF surface staining panel included the following 33 markers: CD45, HLA-DR, CD19, CD3, CD4, CD8, CD14, CCR6, CD11a, Cd123, CCR5, CD7, ICOS, CD25, CD57, CD45RA, CD163, PD-1, PDL-1, CXCR3, CCR4, CCR7, CD28, CTLA4, CD11c, CD56, CD45RO, CD44, CD27, CD138, CD38, CD-127 and CD16. Data processing and analysis was performed using Cytobank. Live cells were identified based on Pt195 and Ir193 staining. Myeloma cells and CD45- cells were excluded and only CD45+ cells were used for subsequent analyses. Single-cell data were downsampled using VisNE, a permutation of t-Distributed Stochastic Neighbor Embedding (tSNE) and clustered using CITRUS (using 10,000 events per sample with a minimum cluster size of 1%). A Significance analysis of microarrays (SAM) analysis was performed to ascertain differences between groups. Significance was inferred for a false discovery rate <1% . All CITRUS analyses were repeated at least 3 times and only clusters found to differ consistently across runs were considered. RESULTS: The proportions of immune subsets identified to vary by CITRUS before and after induction therapy and ASCT for MM and AL are shown in the table. No differences were identified between MGUS, SMM and NDMM. The proportion of a subset (369850) of CD14+/C16- monocytes, a group of cells shown to correlate positively with survival and response to therapy in solid malignancies, increased after induction therapy in MM. Naïve B cells increased dramatically post ASCT in both AL (429918) and MM (369948), consistent with expected immune reconstitution patterns, although a CCR6+ B Cell subset (429940) shown to mediate effective antibody responses, decreased (in grey). A subset (369980) of functionally exhausted (PD1/CTLA4+) central memory (CM) CD4 T cells decreased after induction therapy in MM but recovered early post ASCT whereas a CM CD4+ subset (369986) lacking major activation markers (CD28, CD25) decreased gradually with therapy and post ASCT. A subset (369953) of naïve CD8 T cells shown to be actively recruited in tumor sites (CXCR3+) decreased after ASCT. CD57+ senescent effector memory (EM) CD8 T cells (369962) decreased with induction therapy but recovered post ASCT. EM CD8 T cells associated with long term immune memory (CCR5+, CD127+, cluster 369959), also decreased after ASCT. LIMITATIONS: Include a) No barcoding b) batch effects were difficult to avoid when processing large number of samples c) use of cryopreserved samples d) need for downsampling to cope with the computational burden. The latter could have been one of the reasons we did not identify any differences between MGUS, SMM and MM. At the time of the meeting we will present confirmatory analyses using conceptually different methods of clustering and of performing across group comparisons as well as analyses that do not include downsampling. CONCLUSIONS: Mass cytometry can provide a more granular view of the bone marrow immunome in plasma cell dyscrasias. Novel agent induction therapy can create an immunologically favorable anti-tumor microenvironment although, in some cases, these favorable immunomic shifts are temporarily reversed by ASCT. Confirmatory analyses, baseline immune profiles, comparisons with healthy donors and correlation with other clinically relevant patient characteristics and outcomes will be reported at the meeting.
Dispenzieri:Celgene, Takeda, Prothena, Jannsen, Pfizer, Alnylam, GSK: Research Funding. Kumar:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal